MedaSystems for
Cures Act Compliance
A solution for pre-clinical communication & compliance
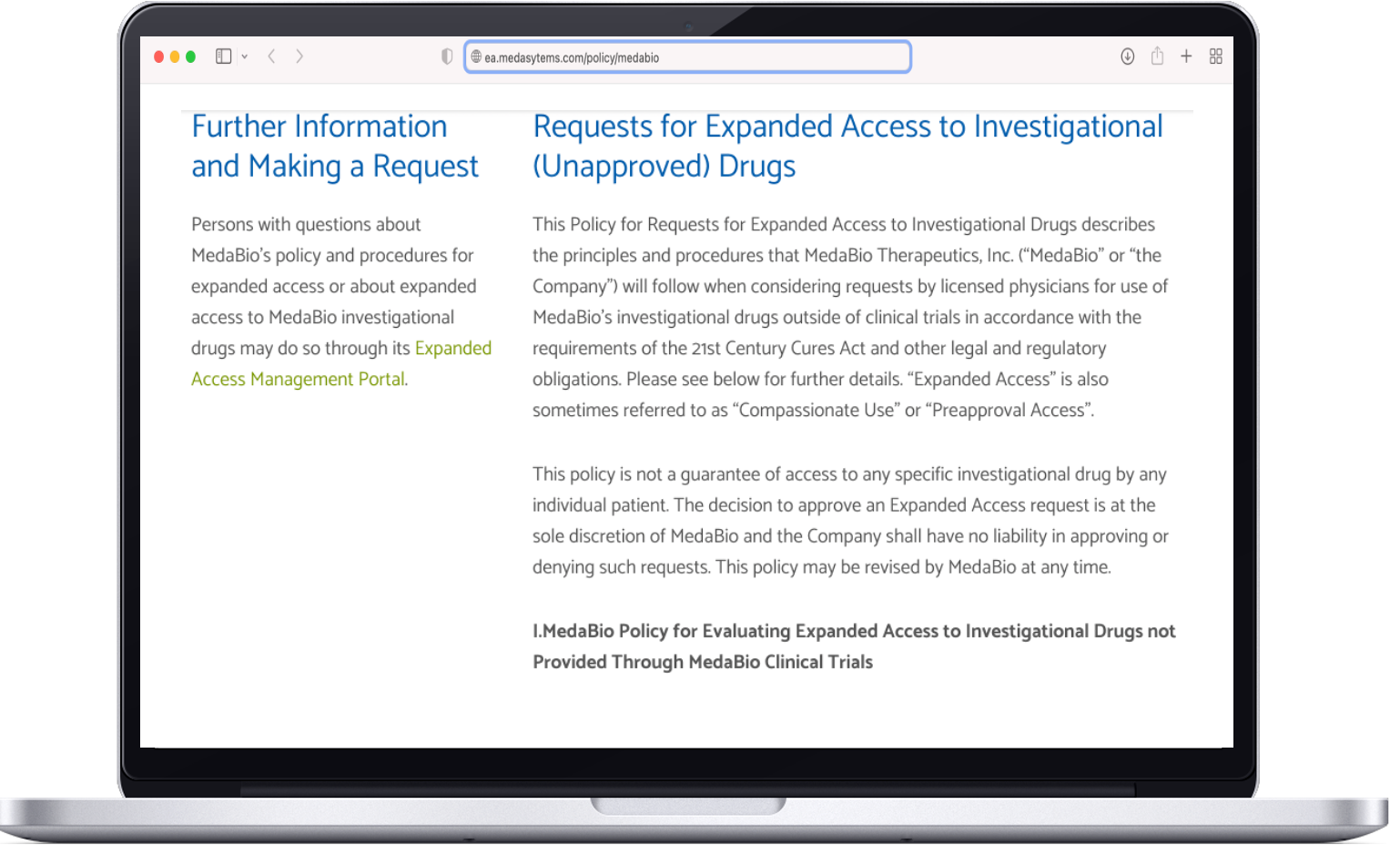
Expanded Access policy
Template policies, including FDA-suggested language
Enumerate criteria for making decisions
Provide contact information
Outline request and adjudication process
Physician request form
Custom fields are based on needs
Avoid receiving patient information via non-secure email
Optional patient-facing inquiry form
Respond to requests
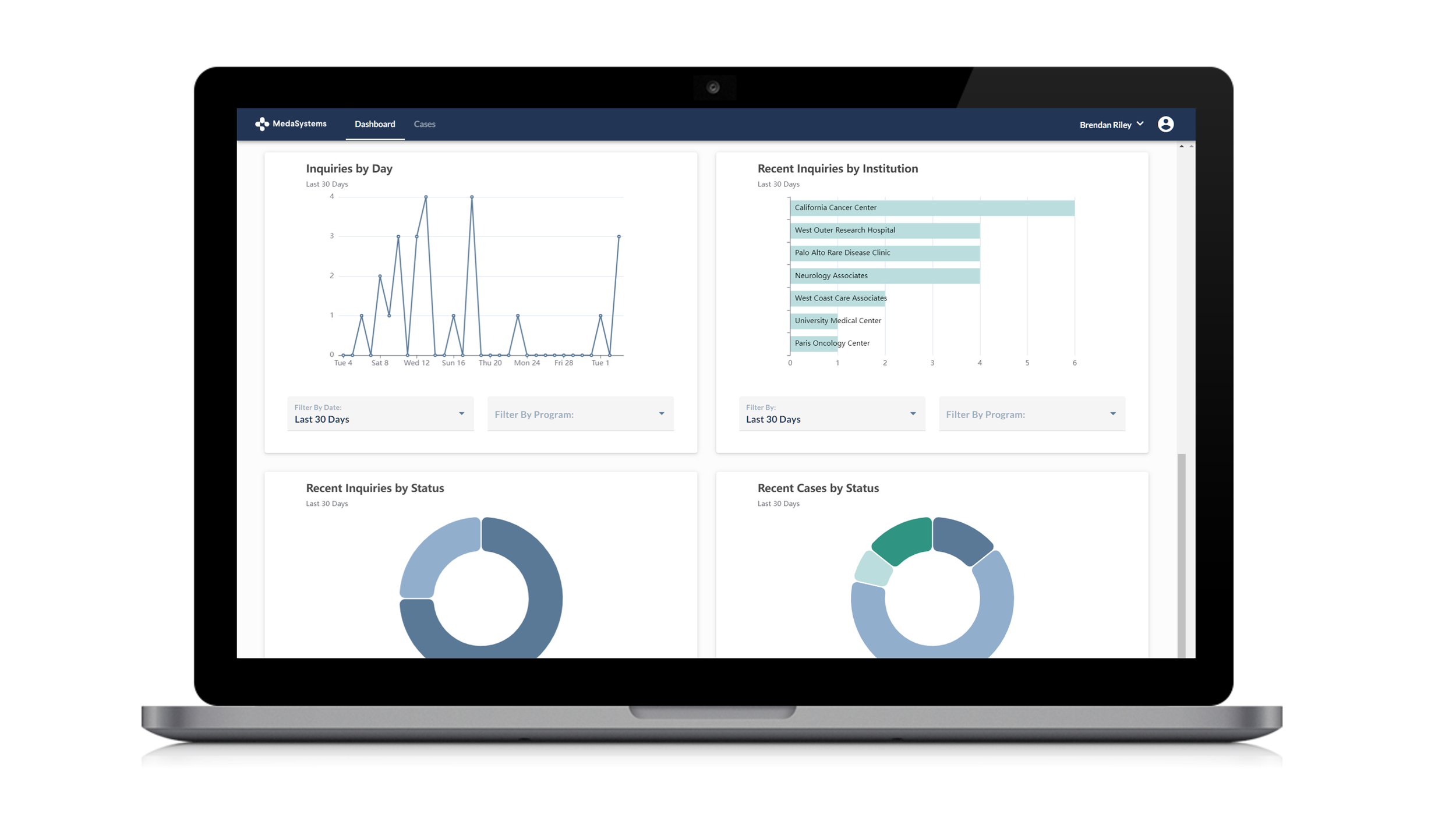
Analyze interest by geography and institution
Connect eligible patients with clinical trials
Ensure compliance with global information security standards including HIPAA and FDA 21 CFR Part 11
Exportable audit trail of all communications
Comply with FDA requirements for communication
FDA requires life science companies in Phase 2 or 3 clinical trials to publish an Expanded Access policy, including providing a way to communicate with the company and an overview of how requests are adjudicated.
MedaSystems Compliance module allows companies to:
Develop a policy
Post the policy and the process for making requests
Securely receive requests in an auditable, compliant format
Gain market insights in advance of commercialization
Seamlessly transition to the full MedaSystems platform if Expanded Access becomes a part of the company’s strategic direction
Why do you need an Expanded Access policy on your website?
The 21st Century Cures Act requires companies developing investigational therapies to publish their expanded access policy. A recent study estimates that 90% of companies are not yet fully compliant.
All policies, even if Expanded Access is not offered, must include:
Contact information and request process
Criteria for evaluating requests
Expected time to acknowledge receipt
Even if the company is not offering Expanded Access, proactively explaining why the company may not be able to accommodate requests provides transparency and responsiveness to patient inquiries.
Request our white paper to learn more about requirements specific to the United States.