MedaSystems for Investigator-Initiated Studies
Simplify IIS Management with a Platform That Scales with You
Investigator-Initiated Studies (IIS) are vital for advancing evidence generation, but managing proposals, approvals, and study tracking can often be complex and time-consuming. MedaSystems offers an intuitive, cloud-based platform designed to streamline the IIS process—from submission to study closure—enabling collaboration, operational excellence, and actionable insights.
Why MedaSystems?
Seamless Collaboration Across Teams
Facilitate real-time collaboration among researchers, sponsors, and reviewers, reducing redundancies and accelerating decisions.Unified Workflow Management
Standardized workflows guide users through every stage, from proposal submission to study closure, with the flexibility to align processes to organizational SOPs.Scalable and Flexible
Whether your IIS program involves local or global studies, MedaSystems grows with you, maintaining visibility and ensuring efficiency as the program expands.Actionable Insights and Reporting
Real-time dashboards and reports make it easy to track submissions, study progress, and portfolio performance, enabling you to align IIS activities with broader medical and strategic objectives.
Transparent Review and Approvals
Equip reviewers with tools to access materials, leave comments, and record decisions in a collaborative, centralized environment.
Automated notifications ensure smooth communication and keep everyone on track.
Enhanced Portfolio Management
Align IIS projects with broader organizational goals, evaluate data gaps, and assess the overall impact of your portfolio.
How MedaSystems Supports IIS Success
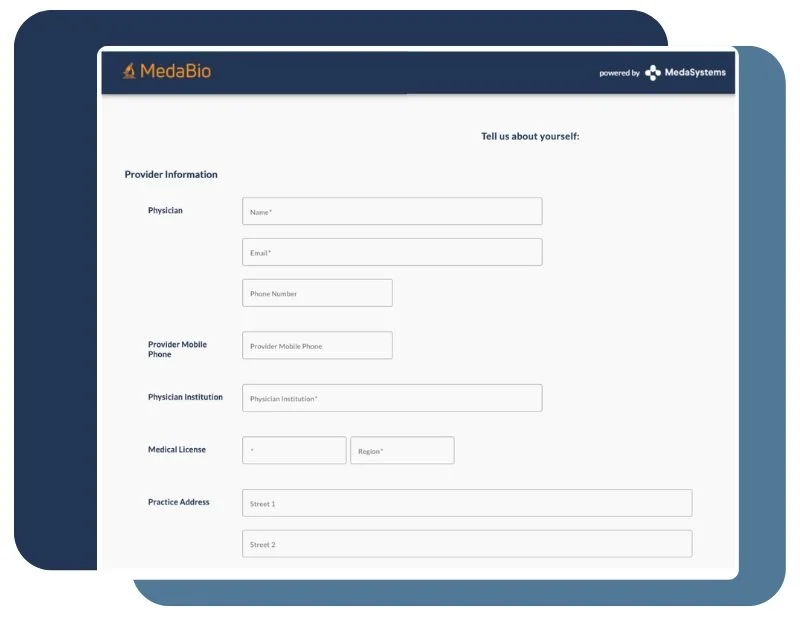
Streamlined Proposal Submission
Provide researchers with a centralized portal to submit complete applications, including hypotheses, budget, and timelines.
Allow researchers to modify or update submissions as needed, ensuring accuracy.
Efficient Study Tracking and Reporting
Use real-time dashboards to monitor active studies, pending submissions, and key performance indicators like approval rates and milestones.
Track budgets, manage drug supply, and maintain visibility across studies.
All the tools you need in one collaboration platform
-
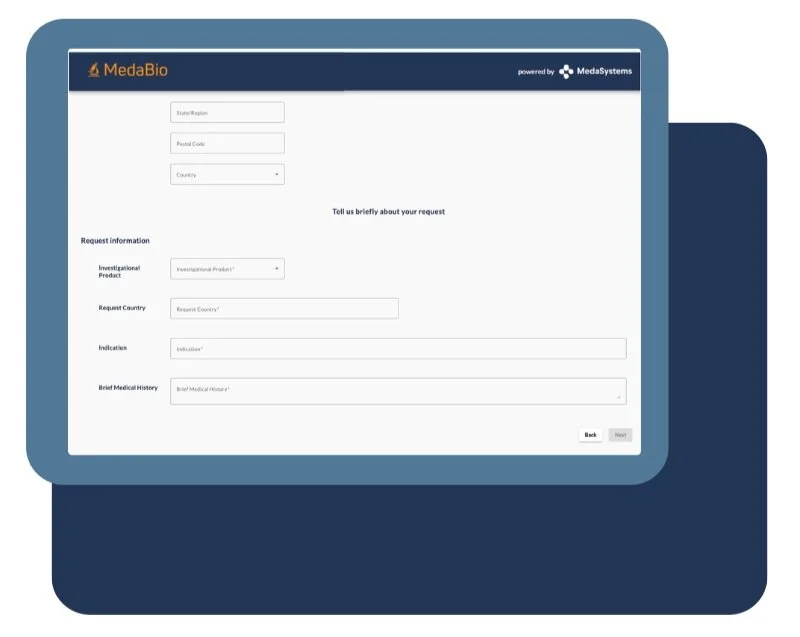
Submission
Tailored forms capture essential proposal details, including hypotheses, budgets, and timelines, ensuring researchers provide complete information.
-
Workflows
Configurable workflows guide submissions, approvals, and study tracking, creating a consistent and repeatable process.
-
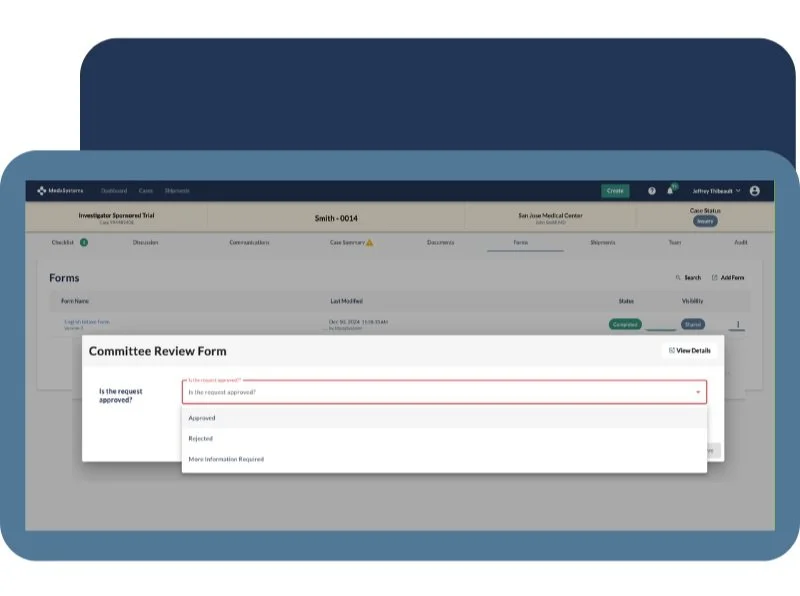
Review
Enable reviewers to leave comments, collaborate with colleagues, and make informed decisions—all in a single environment.
-
Analyze
Real-time dashboards help track performance metrics, identify trends, and evaluate outcomes at both the study and portfolio levels.